APIs for chemistry and biology
It’s interesting to view chemistry and biology through the lens of software and APIs. Wikipedia defines an application programming interface as a way for software programs to communicate with each other via well defined rules (protocols), so that a service provider, whose API is being called, can provide a service while hiding the details of the implementation so that the service consumers don’t need to worry about those details. This enhances modularity and composability of a software system.
Similar principles apply to chemistry and biology! Certain chemical functional groups can be viewed as a type of API because we see the same functions being leveraged by many processes in a modular, composable way. A great example is a phosphate group, a phosphorus atom bonded to four oxygen atoms:
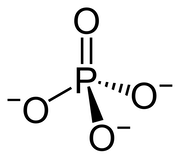
In molecules such as ATP that contains phosphate groups, the linkage between two phosphorus atoms and the connecting oxygen atom is kinetically stable (e.g., the ATP molecule is stable in the cell), but thermodynamically unstable (it is energetically favorable to break the bond to replace with a more thermodynamically stable configuration and thus release energy, e.g, hydrolysis of ATP). Also, a phosphate group tends to be negatively charged.
Together, these factors enable a large variety of biochemical “use cases” where energy needs to be controllably stored or released (ATP as the “energy currency of cells”), or charge needs to be controllably added or removed (kinase/phosphatase to add or remove charge to proteins and thereby switch on/off their functions). And phosphate groups are a sort of “modular molecular API” that is used by these biochemical processes.
Here is a great discussion of the importance of phosphates to life as we know it. Hint: be sure to read until the end of the page.
Useful Links
- Tim Soderberg’s online text, Organic Chemistry with a Biological Emphasis: Chapter on Phosphate Transfer Reactions